Constitutional Isomerism
Constitutional isomers, also known as structural isomers, have the same molecular formula but different connectivity. The compounds can only be interconverted by breaking and reforming one or more bonds connected to the chiral center(s) with different spatial orientation.1 Symmetrical monomers without a chiral center such as ethylene and tetrafluoroethylene can be joined together in only one way. Monosubstituted (vinyl) monomers, on the other hand, may be joined together in three distinct ways for adjacent repeat units:
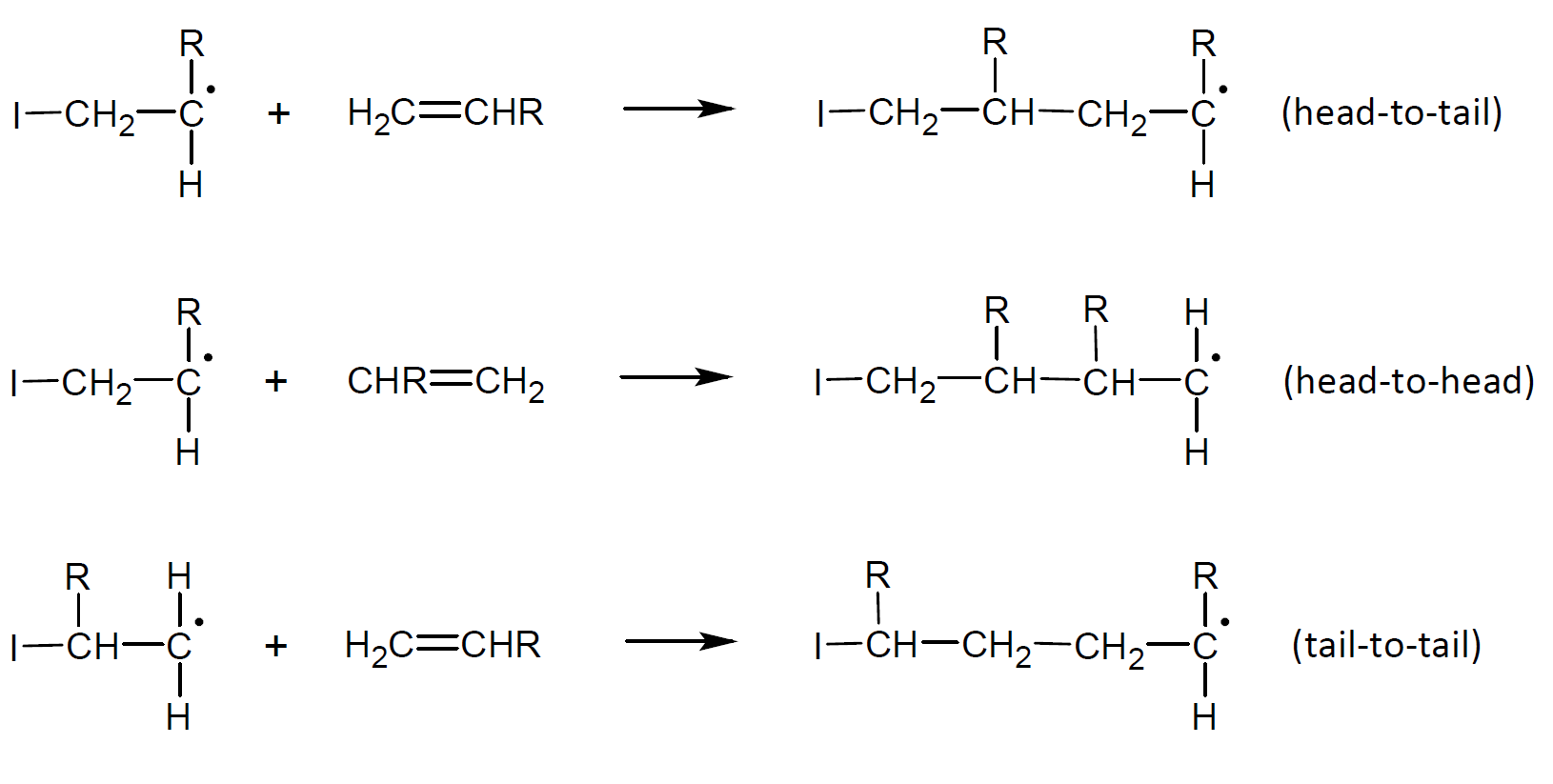
In the case of free radical or ionic polymerization, the mode of monomer addition to the chain growth center is mainly head-to-tail (or 1,3-placement), so that the pendant groups (and chiral centers) are on every other carbon atom in the polymer backbone. Polymers that grow predominantly in this fashion include propylene, styrene, vinyl chloride, acrylonitrile and (meth)acrylates. This arrangement is typically favored due to resonance stabilization and steric effects in the growing chain whereas regio-specific head-to-head polymers can only be prepared by post modification of diene polymers or alternating copolymers, for example by hydrogenation of 1,4-polydienes such as 1,4-poly(2,4-hexadiene) and 1,4-poly(2,3-dimethyl-1,3-butadiene).2-4
The percentage of head-to-tail and head-to-head arrangements in the polymer will depend on the reaction conditions and polymerization method. A higher proportion of head-to-head placement in free radical polymerization can be expected with increasing temperature5 whereas polyolefins produced with Ziegler-Natta catalysts are principally linear, regular head-to-tail polymers.6,7 Typically, only a small portion of not more than 1 - 2% (and in a few cases up to 20%) will be in head-to-head or head-to-tail placement.5,8 Polymer synthesized by a cationic mechanism, on the other hand, do not possess a mainly head-to-tail sequence but are often of irregular structure with a larger head-to-head and tail-to-tail portion in the polymer chain which can easily account for 10% to 30% of the polymer.9
Step-growth polymerizations of bifunctional monomers (i.e. A-R'-A + B-R''-B) are generally not regioselective and yield random polymers with head-to-tail and head-to-head structures more or less randomly arranged in the polymer chain because the two functional groups in the monomer molecules that undergo step-growth polymerization do not differ enough in reactivity and steric hindrance.10
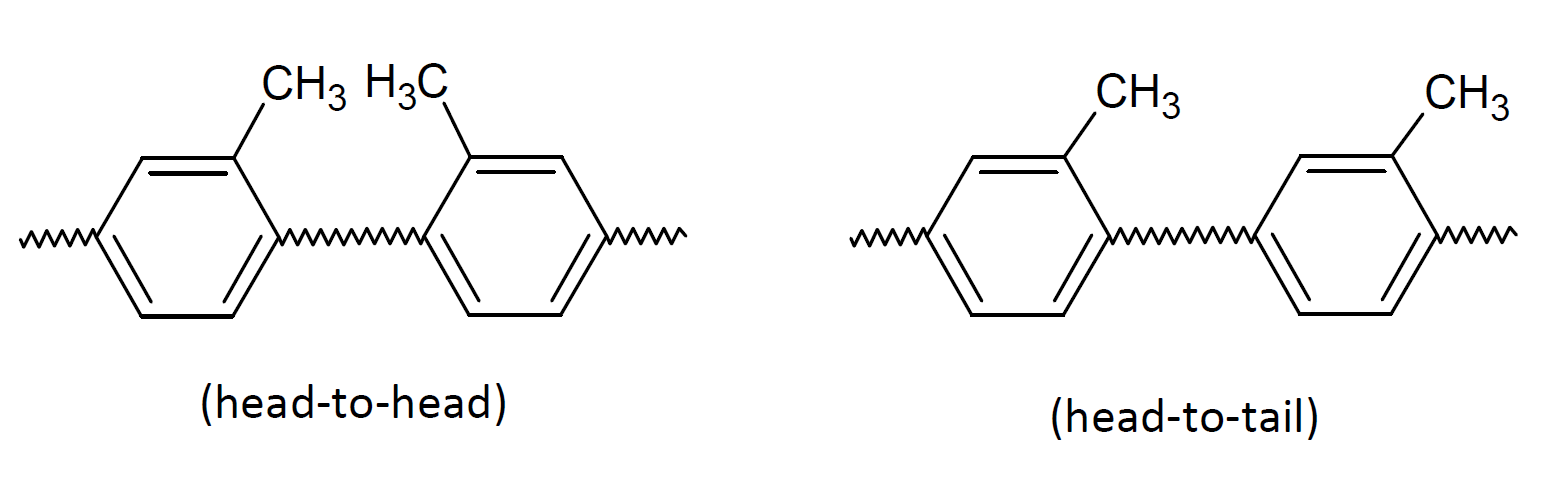
The effect of structural arrangements of the repeat units on the polymer properties such as crystallinity and glass transition temperature (Tg) will largely depend on the substituents which control the flexibility of the polymer. In the case of polypropylene, a higher proportion of head-to-head arrangement will lower the Tg and in the case of polystyrene it will not affect the Tg much.9
References and Notes
Conformational isomerism, on the other hand, is defined as the spatial arrangements of bonds in the molecule which arise from rotations about single bonds whereas configurational isomerism describes stereoisomers that cannot be converted into one another by rotations about single bonds.
- Y. Zhang, J. Li, X. Li,J. He, Macromolecules, 47, 18, 6260-6269 (2014)
- S. Arichi, M.Y. Pedram, J.M.G. Cowie, Euro. Poly. J, Vol 15 (2), pp. 107-112 (1979)
- S. Grossman, A. Stolarczyk, O. Vogt, Monatshefte fuer Chemie 112, 1279-1296 (1981)
- W.-F. Su, Principles of Polymer Design and Synthesis, Vol 82, pp 137-183 (2013)
G. Natta, P. Pino and G. Mazzanti; US Patent 3,715,344; Regular Linear Head-to-Tail Polymerization of Certain Unsaturated Hydrocarbons.. (1973)
G. Natta, P. Pino and G. Mazzanti; US Patent 3,715,344; Regular Linear Head-to-Tail Polymerization of Certain Unsaturated Hydrocarbons.. (1973)
Peter Nesvadba, Radical Polymerization in Industry in Industry in Encyclopedia of Radicals in Chemistry, Biology and Materials, John Wiley & Sons 2012
M.T. Malanga, Synthesis and Characterization of Head-to-Head Polyisobutylene and Related Polymers, Doctoral Dissertations (1982)
Yokoyama and Yokozawa reported the synthesis of well-defined aromatic polyamides, polyesters, and polyethers via substituent effect-assisted chain-growth condensation polymerization. Their method has been employed for the synthesis of aromatic polyamides with well-defined structure.11
- A. Yokoyama and T. Yokozawa, Macromolecules 40 (12), pp. 4093-4101 (2007)